Abstract
About 10-30% of myelofibrosis patients who undergo allogeneic hematopoietic cell transplantation will eventually experience relapse within 5 years. While significant improvement has been achieved in stratifying patients according to their risk for posttransplant mortality, preventing and treating relapse remains an unmet clinical need. Notably, no standard of care exists for treatment of relapsed myelofibrosis.
On option in the transplant setting (mainly established in CML and AML) is transferring donor T cells to reinforce a graft-versus-leukemia (GVL) effect targeting the recurring malignant cells. Previous small sample studies also indicated a strong graft-versus-myelofibrosis effect of donor lymphocyte infusion (DLI). Additionally, molecular monitoring posttransplant may further help to target outcomes and identify cohorts with distinct risk for and outcome after relapse receiving DLI.
Here, we evaluated the largest cohort to date of myelofibrosis patients receiving DLI. The aim of this study was to induce molecular complete remission (CR) and to evaluate associations with overall outcomes.
Patients were eligible to receive DLI if they were free of GVHD and immunosuppressive therapy. If no GVHD or response occurred within 6 weeks, another escalating dose (half-log) of DLI was administered. Highly sensitive chimerism and molecular monitoring with developed driver mutation-specific PCRs for JAK2, CALR and MPL was done every month (until day 360 posttranspant) and with routine visites thereafter
We present results of 57 genetically annotated myelofibrosis patients who received donor lymphocyte infusion (DLI) for persistent disease (n=20), molecular relapse (n=17), or hematologic relapse (n=20), aiming to achieve molecular complete remission (CR). A total of 143 DLI procedures were given (range, 1-5 cumulative DLIs). Median CD3+ starting dose of first DLI was 1 x106 (range, 1 x105-1 x107), escalated by half-log after at least 6 weeks if no response nor graft-versus-host disease occurred.
Overall molecular CR rate was 70% (n=38) and seemed to be higher for patients who received first DLI for molecular relapse (88%, 15 out of 17). For each DLI, molecular CR was achieved in 40% (23 out of 57) after first, in 44% (17 out of 39) after second, in 32% (8 out of 25) after third, in 470% (7 out of 15) after fourth, and in 43% (3 out of 7) after fifth DLI. In terms of acute GVHD, 15 overall cases occurred after a total number of 143 procedures (11%) and appeared to be associated with response.
Of 22 patients who achieved initial molecular CR after first DLI, 11 patients (50%) received subsequent DLIs, of whom 4 patients initially received first DLI for hematological relapse, 4 patients for molecular relapse, and 3 patients for disease persistence. Relapses after first DLI were hematological in 8 patients (73%) and molecular in 3 patients (27%), and second DLI was given within a median of 10 weeks (range, 6-323 weeks) from first DLI. Of those, 9 patients could be eventually converted with subsequent DLIs into durable molecular CR (82%) with a median of 7 years after last DLI.
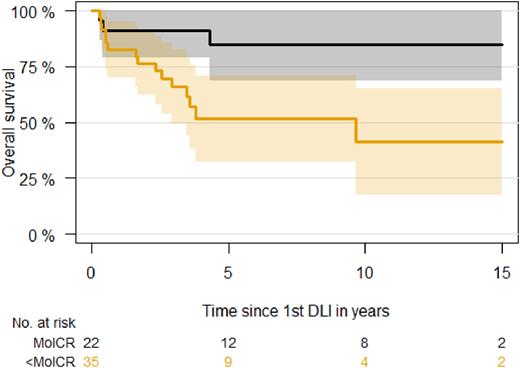
Overall outcome was driven by response to first DLI. Five-year overall survival after first DLI was 85% (95% CI, 67-100%) for patients with molecular CR versus 52% (95% CI, 33-71%) for patients with less than molecular CR. Achievement of molecular CR after first DLI was associated with 78% reduced risk of death (P=0.02). All patients who relapsed after initial molecular CR after first DLI (50%, n=11) were alive at last follow-up.
Multivariable analysis on overall survival and disease-free survival, adjusting for time-dependent and continuous effects of total number of DLIs, showed significantly reduced risk of death for patients with molecular CR after first DLI (with patients achieving less than molecular CR as reference), with hazard ratio of 0.22 (95% CI, 0.06-0.80; P=0.02). Similar results were retrieved for risk of death or relapse/disease persistence after last DLI, with a hazard ratio of 0.14 (95% CI, 0.05-0.39; P<0.001). No significant effect of number of DLIs or indication for first DLI were identified.
Targeting molecular CR after DLI is essential to achieve excellent long-term survival in persistent or relapsed myelofibrosis, and DLI together with molecular monitoring should be standard of care for relapsed myelofibrosis.
Disclosures
Ayuk:Gilead: Honoraria; Celgene/BMS: Honoraria; Novartis: Honoraria; Mallinckrodt/Therakos: Honoraria, Research Funding; Miltenyi Biomedicine: Honoraria; Janssen: Honoraria; Medac: Honoraria; Takeda: Honoraria. Kroeger:Novartis: Honoraria; Kite/Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal